You reach for it almost daily, a humming box of convenience ready to reheat leftovers or pop some corn. It’s your microwave oven. You press a button, hear the familiar whir, and moments later, your meal is warm. But have you ever stopped to think about the actual magic happening inside? It’s not simply tiny invisible flames, despite what your imagination might conjure.
Many people wonder about the science behind this everyday appliance. How does this box take something cold and make it piping hot so quickly? The secret lies in a fascinating interaction between specific types of energy waves and the very molecules that make up your food. If the quick rundown below feels like a sudden burst of understanding, much like a warm snack from the microwave, you might enjoy seeing the concept explained visually:
Table of Contents
The Star Player: The Magnetron
At the heart of your microwave oven is a component you might never see, but it’s the key to everything: the magnetron. Think of the magnetron as a specialized vacuum tube designed to generate a specific type of electromagnetic wave. Unlike the gentle waves that carry radio signals or the visible light waves that illuminate your room, the magnetron produces waves in the microwave portion of the electromagnetic spectrum.
These microwaves are directed into the cooking cavity of the oven. This cavity is essentially a Faraday cage, designed to trap the microwaves inside, bouncing them around to ensure they interact with the food.
Microwaves Meet Molecules: The Water Dance
Here’s where the real heating action begins. While microwaves can interact with other molecules like fats and sugars, their primary target – and the reason microwave ovens are so effective – is water. Most of the food we eat contains a significant amount of water.
Water molecules (H₂O) are polar molecules. This means they have a slight positive charge on one side (where the hydrogen atoms are) and a slight negative charge on the other side (around the oxygen atom). They are like tiny magnets with a positive and negative pole.
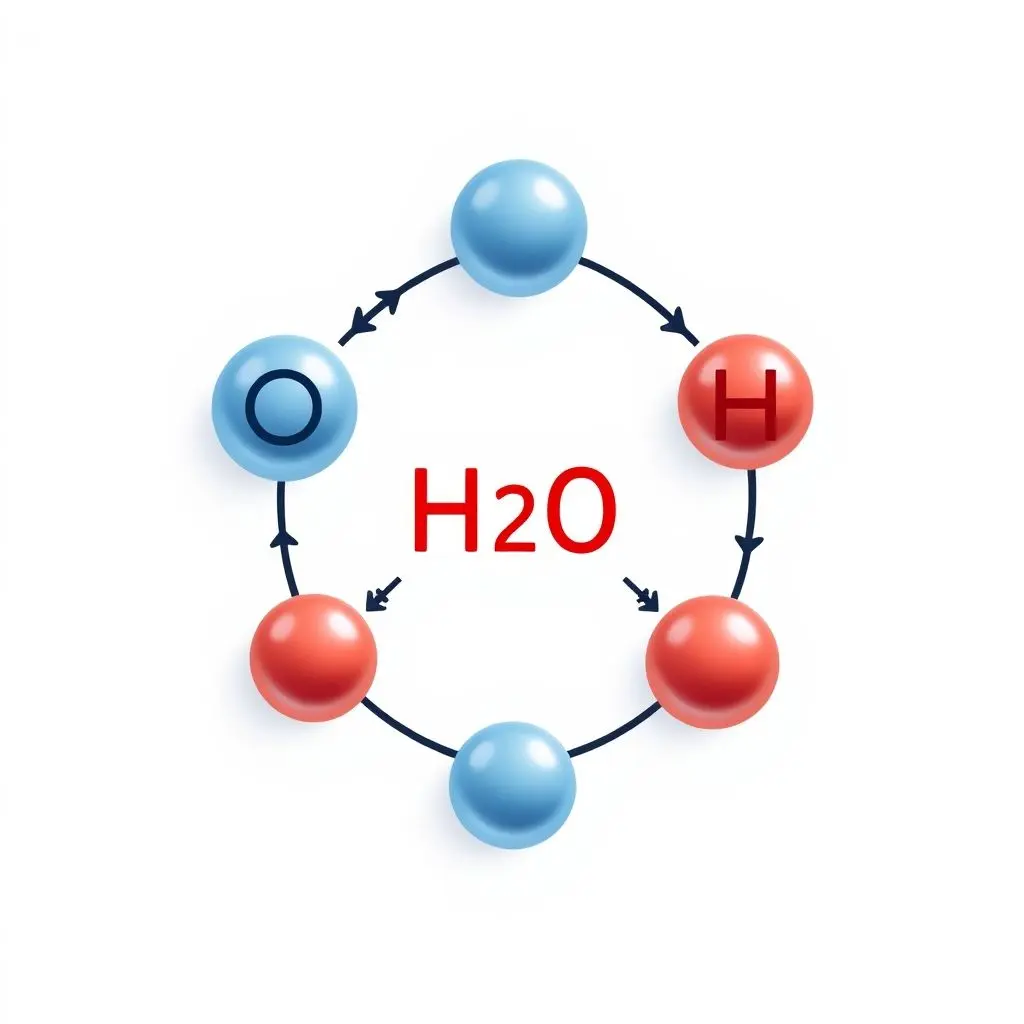
When the microwaves enter the oven cavity and encounter these water molecules, things get exciting. Microwaves are oscillating electromagnetic fields. As the microwave field alternates rapidly, it exerts a torque on the polar water molecules, causing them to try and align with the field’s direction. Since the field is constantly flipping polarity (billions of times per second!), the water molecules are forced to rotate and vibrate at incredibly high speeds to keep up.
Imagine a room full of people forced to constantly twist and turn. As they jiggle frantically, they bump into each other and the surrounding molecules (fats, sugars, proteins). This intense molecular friction and agitation generate kinetic energy, which we perceive as heat. The more the molecules vibrate and collide, the hotter the food becomes.

Penetration, Not Just Surface Heat
A common misconception is that microwaves heat food ‘from the inside out’. While they don’t just heat the surface like a traditional oven or stove, the heating isn’t strictly from the absolute center either. Microwaves penetrate the food (typically a few centimeters, depending on the food’s composition and density), exciting water molecules throughout that volume. Heat is then transferred from these excited water molecules to their less-excited neighbors through conduction.
In thicker foods, the center might heat up as energy conducts inward from the microwave-heated outer layers, which can give the *impression* of heating from the inside. However, the primary interaction happens where the microwaves penetrate.
Why the Turntable? Why Uneven Heating?
If microwaves are bouncing all around the cavity, why does your food sometimes end up with hot and cold spots, and why the spinning plate?
The bouncing microwaves create standing wave patterns within the oven cavity. This means some areas have more intense microwave energy (hot spots) and others have less (cold spots). The turntable helps to move the food through these different energy zones, allowing for more even exposure to the microwaves and thus more uniform heating. Without it, you’d consistently get certain parts of your food much hotter than others.
Are Microwaves Safe?
Concerns about microwave radiation and safety are common. Rest assured, microwave ovens are designed with safety in mind. The metal mesh in the door and the oven walls act as a Faraday cage, effectively containing the microwave radiation within the cavity when the door is closed and the oven is operating. As soon as the door is opened, safety interlocks immediately shut off the magnetron, stopping the production of microwaves.
The microwaves generated are non-ionizing radiation, meaning they don’t have enough energy to strip electrons from atoms and damage DNA, unlike X-rays or gamma rays. The energy they impart simply causes molecules to vibrate and heat up. Once the microwave is off, there is no residual radiation in the food or the oven.
Regarding nutrient loss, studies have shown that microwave cooking is often *better* at preserving nutrients than traditional methods like boiling, which can leach water-soluble vitamins. The shorter cooking time and use of less water in microwaving help retain more vitamins and minerals.
Frequently Asked Questions About Microwaves
Let’s tackle some common queries:
Q: Can I put metal in a microwave?
A: Generally, no. Metal is reflective to microwaves. Flat metal can cause the waves to bounce erratically, potentially damaging the magnetron. Pointed or crumpled metal (like foil) can concentrate the electric field, leading to arcing or sparking, which is a fire hazard. Some specially designed metal microwave-safe containers exist, but assume metal is off-limits unless specifically labeled otherwise.
Q: Does microwaving food make it radioactive?
A: Absolutely not. Microwaves are non-ionizing radiation. They heat food by exciting molecules, but they do not alter the food’s atomic structure or leave any residual radiation behind. It’s like warming your hands by rubbing them together – your hands get warm from friction, but they don’t become radioactive.
Q: Does microwaving kill nutrients?
A: No, in fact, it can often preserve more nutrients than other cooking methods. Nutrient loss during cooking is primarily due to time, temperature, and the amount of water used. Since microwaving is typically faster and uses less water, it can minimize the degradation and leaching of heat-sensitive and water-soluble vitamins.
Q: Why do some foods get hotter than others?
A: This is mainly due to differences in water content, density, and composition. Foods with higher water or fat content tend to heat faster in a microwave because these molecules interact strongly with microwaves. Dense foods heat slower as the energy takes longer to conduct throughout the volume.
Q: How deep do microwaves penetrate food?
A: The penetration depth varies depending on the food and the microwave frequency (usually 2.45 GHz in home ovens). Typically, it’s a few centimeters (around 1 to 1.5 inches). Beyond this depth, heat is transferred by conduction from the outer heated layers.
The Everyday Marvel
The humble microwave oven, sitting on your counter, is a marvel of applied physics. It leverages the simple, elegant principle of using specific electromagnetic waves to target and excite water molecules within your food. This rapid molecular dance party generates the heat that warms your meals. It’s a testament to how understanding fundamental properties of energy and matter can lead to incredibly convenient technology that simplifies our daily lives.