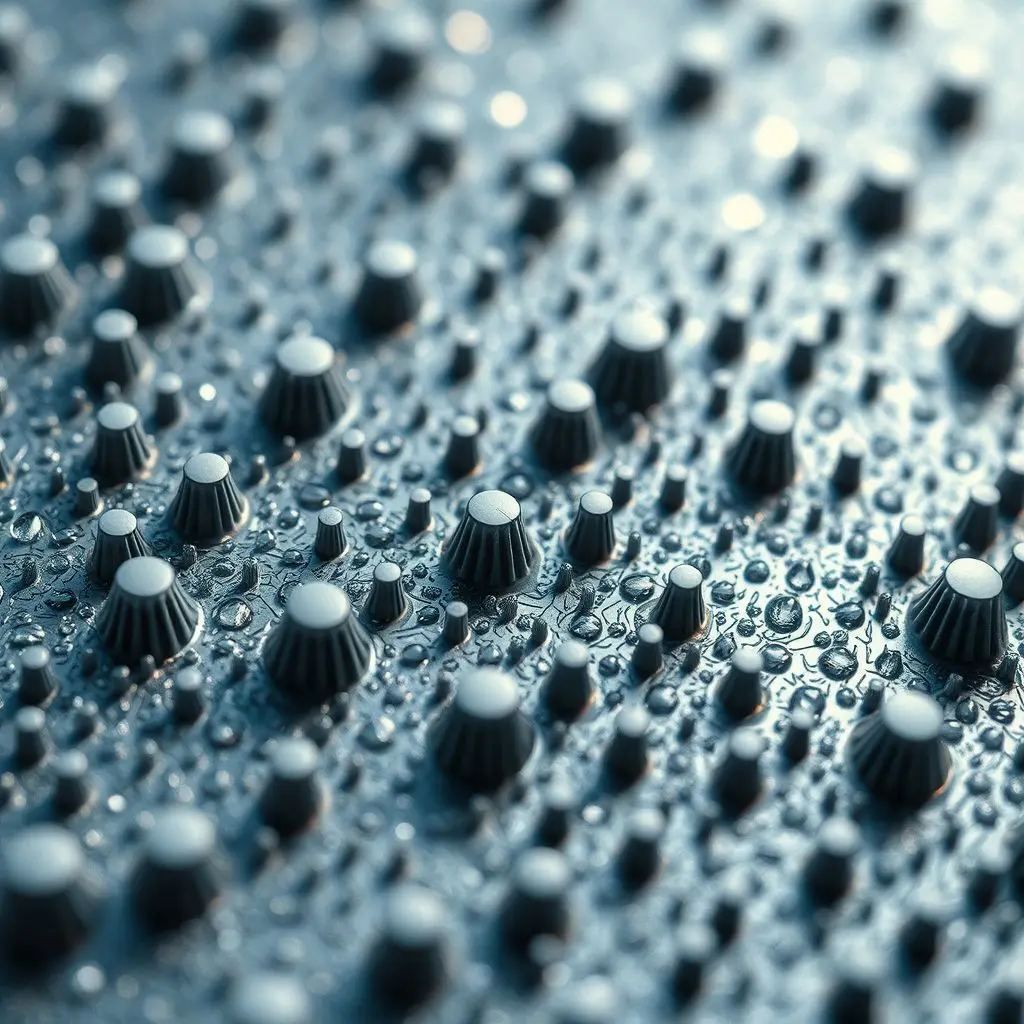
Ever witnessed water defy gravity, literally bouncing off a surface like a tiny, liquid trampoline artist? Or perhaps a raindrop making a dramatic disappearing act the moment it touches a screen? This isn’t some mystical illusion; it’s a brilliant display of science at work, powered by what we call hydrophobic coatings.
Imagine a microscopic, invisible force field shielding your most prized possessions, your clothing, or even parts of your home from the relentless assault of water. That’s precisely what these remarkable coatings do. Instead of letting moisture soak in, they actively push it away, compelling water to bead up and roll off. The secret lies in creating surfaces with incredibly low surface energy, often achieved through intricate, sometimes nanoscale, structures that ingeniously mimic nature’s own designs – like the awe-inspiring lotus leaf effect.
Table of Contents
What Exactly Are Hydrophobic Coatings?
At its core, “hydrophobic” literally translates to “water-fearing.” So, a hydrophobic coating is a material applied to a surface that makes it repel water. But it’s not just about simple repellency; it’s about altering the fundamental interaction between the surface and water molecules.
To understand this, we need to talk about contact angle. When a droplet of liquid sits on a surface, the angle formed between the edge of the droplet and the surface is the contact angle. For a surface to be considered hydrophobic, this angle typically needs to be greater than 90 degrees. The higher the contact angle, the more water-repellent the surface. When the contact angle exceeds 150 degrees, we enter the realm of superhydrophobicity, where water droplets appear almost spherical and roll off with minimal effort, carrying dirt and dust with them – a phenomenon often referred to as “self-cleaning.”
The Science Behind the Magic: From Lotus Leaves to Nanotech
Nature has always been the ultimate engineer, and for hydrophobic surfaces, the humble lotus leaf serves as the supreme inspiration. If you’ve ever observed a lotus leaf after a rain shower, you’d notice it remains perfectly dry, with water droplets pearling up and rolling away. This isn’t because the leaf is covered in a waxy, smooth layer. Quite the opposite.
Under a microscope, a lotus leaf reveals a rough, hierarchical structure of microscopic bumps, each covered in even tinier, nanoscale, wax crystals. This combination of roughness and low surface energy wax creates an air pocket between the water droplet and the leaf surface. Water, preferring to stick to itself rather than penetrate these air pockets, minimizes contact with the surface, resulting in that characteristic high contact angle and the water-shedding “lotus effect.”
Scientists and engineers have spent decades perfecting ways to replicate this natural wonder. Modern hydrophobic coatings achieve this by:
- Creating Roughness at the Nano-level: By depositing materials in a way that forms tiny pillars, ridges, or other textured patterns on the surface, similar to the lotus leaf.
- Using Low Surface Energy Materials: Incorporating substances like fluoropolymers (think Teflon® or PTFE), silicones, or specific waxes that inherently have a low affinity for water molecules.
When water touches such a surface, it lacks sufficient energy to penetrate the microscopic textures and instead sits atop these structures, supported by a layer of trapped air. This minimal contact area is what forces the water to bead up and roll off effortlessly.
Before we dive deeper into the incredible applications of this technology, take a moment to watch this short video that visually explains the magic of hydrophobic coatings:
How Hydrophobic Coatings Work: A Deeper Dive
It’s all about the interplay of intermolecular forces. Water molecules are highly polar and exhibit strong cohesive forces, meaning they prefer to stick together. When water encounters a surface, two sets of forces come into play: cohesive forces (water-water attraction) and adhesive forces (water-surface attraction).
- On a hydrophilic (water-loving) surface, adhesive forces are stronger than cohesive forces. Water spreads out, wets the surface, and forms a low contact angle.
- On a hydrophobic (water-fearing) surface, cohesive forces within the water droplet are much stronger than the adhesive forces between the water and the surface. The water molecules “prefer” to stay together, minimizing contact with the surface, resulting in a high contact angle and the characteristic beading.
The textured surface of a hydrophobic coating enhances this effect by reducing the actual solid-liquid contact area. Even if the coating material itself isn’t perfectly water-repellent, the combined effect of its intrinsic low surface energy and the engineered roughness creates a barrier of trapped air, preventing water from “wetting” the surface. This is known as the Cassie-Baxter state of wetting.
This ingenious mechanism isn’t just a laboratory curiosity; it’s a foundational principle driving countless innovations.

Beyond Just “Waterproof”: Applications Across Industries
The applications of hydrophobic coatings stretch far beyond merely keeping things dry. They offer enhanced durability, cleanliness, and functionality across a vast array of sectors.
Consumer Electronics: The Unseen Shield
Remember that feeling of dread when a drink spills near your phone? Hydrophobic coatings are increasingly integrated into smartphone screens, smartwatches, and other gadgets. While they don’t make devices truly submersible (that requires comprehensive sealing), they provide an excellent first line of defense against accidental splashes, fingerprints, and smudges, making screens easier to clean and less prone to water spots.
Textiles & Apparel: Stain-Free and Functional Fabrics
From your outdoor gear to your living room sofa, hydrophobic treatments revolutionize textiles. They make clothing resistant to rain, mud, and everyday spills like coffee or wine. Imagine jackets that shed water effortlessly or tablecloths that remain pristine despite enthusiastic eaters. This technology allows fabrics to remain breathable while offering superior stain and water resistance, a significant upgrade from traditional, often less comfortable, “waterproof” materials.

Automotive: Clearer Vision, Cleaner Rides
Hydrophobic coatings applied to car windshields (often marketed as “rain repellents”) cause rainwater to bead up and roll off rapidly, dramatically improving visibility during heavy downpours. This reduces the need for wipers, especially at higher speeds. Beyond windshields, these coatings are used on car paint to protect against water spots, dirt adhesion, and even minor scratches, keeping vehicles cleaner for longer and simplifying washing.
Medical & Healthcare: Sterility and Performance
In medical settings, cleanliness is paramount. Hydrophobic coatings play a crucial role in preventing the adhesion of blood, proteins, and bacteria to surgical instruments, implants, and diagnostic equipment. This minimizes biofouling, reduces the risk of infection, and facilitates easier sterilization. They can also enhance the performance of lab equipment by ensuring precise liquid handling.
Industrial & Construction: Durability and Maintenance
Buildings, bridges, and industrial machinery are constantly exposed to the elements. Hydrophobic coatings can protect surfaces from corrosion, frost damage, and the build-up of dirt, grime, and pollutants. This translates to reduced maintenance costs, extended lifespan of structures, and more aesthetically pleasing urban environments thanks to “self-cleaning” facades.
Marine Applications: Combating Drag and Fouling
For boats and ships, hydrophobic coatings on hulls can reduce drag by creating a smoother interaction with water, potentially leading to fuel efficiency improvements. More importantly, they resist biofouling—the accumulation of microorganisms, plants, and algae on submerged surfaces—which is a major problem for marine vessels, impacting speed and requiring costly maintenance.

Challenges and the Horizon of Hydrophobic Coatings
While the benefits are clear, hydrophobic coatings are not without their challenges. The primary hurdles include:
- Durability: Many early coatings suffered from poor mechanical durability, meaning their water-repellent properties could be lost through abrasion, scratching, or prolonged exposure to harsh conditions. Significant advancements are being made to address this, with new formulations offering much improved robustness.
- Cost: Specialized materials and complex application processes can make some coatings expensive, limiting widespread adoption in certain low-cost applications.
- Scalability: Manufacturing uniform, superhydrophobic surfaces over large areas efficiently and cost-effectively remains a research focus.
- Environmental Impact: Some fluorinated compounds used in certain coatings have raised environmental concerns, driving research into more eco-friendly alternatives.
The future of hydrophobic coatings is incredibly exciting. Researchers are exploring:
- Smart Coatings: Materials that can switch between hydrophobic and hydrophilic states on demand, controlled by light, temperature, or electric fields.
- Self-Healing Coatings: Surfaces that can repair themselves if their water-repellent properties are damaged.
- Omniphobic Coatings: Even more advanced coatings that repel not just water, but also oils, solvents, and other liquids.
- Sustainable Materials: Developing coatings from bio-based or recyclable materials to reduce environmental footprint.
Frequently Asked Questions About Hydrophobic Coatings
- What’s the difference between hydrophobic and waterproof?
- While often used interchangeably, there’s a nuanced difference. “Waterproof” implies a material or product completely prevents water ingress, often through a barrier or sealed construction (like a sealed phone or a rubber boot). “Hydrophobic” describes a surface’s property to repel water. A hydrophobic coating makes a surface water-repellent, causing water to bead and roll off. While it can contribute to waterproofing, a hydrophobic coating alone might not make a device fully “waterproof” if there are unsealed openings.
- How long do hydrophobic coatings last?
- The lifespan varies significantly depending on the type of coating, the surface it’s applied to, and the environmental exposure. For instance, a phone screen coating might last months to a year under normal use, while a car windshield treatment could last several months to a year, and industrial coatings might be designed for years of service. Abrasion, UV exposure, and harsh chemicals can degrade them over time.
- Can I apply a hydrophobic coating myself?
- Yes, many consumer-grade hydrophobic sprays and wipes are available for items like car windshields, shoes, and phone screens. These are generally easy to apply. However, professional applications for industrial or highly technical uses typically require specialized equipment and expertise to ensure proper adhesion, durability, and performance.
- Are hydrophobic coatings safe?
- Most modern hydrophobic coatings for consumer products are considered safe. However, it’s always wise to check the product’s safety data sheet (SDS) and follow application instructions, especially regarding ventilation. Research is also ongoing to develop more environmentally friendly alternatives to older fluoropolymer-based chemistries that have raised some concerns.
Unveiling the Future of Dryness
From a simple water droplet defying gravity to entire buildings shedding grime, hydrophobic coatings are a testament to how deeply we can understand and manipulate the world at the molecular and nanoscale. This isn’t just about keeping things dry; it’s about extending product lifespans, enhancing hygiene, boosting efficiency, and unlocking entirely new possibilities for design and function.
The next time you see water effortlessly glide off a surface, remember: it’s not magic. It’s the remarkable interplay of materials science, biomimicry, and nanoscale engineering, continuing to evolve and reshape our interaction with one of Earth’s most fundamental elements. The quest for the ultimate water-repellent surface is far from over, promising even more jaw-dropping innovations just around the corner.