Ever peered through a windowpane, marveling at the world outside as if the glass weren’t even there? It’s such an everyday occurrence that we rarely stop to think about *why* this seemingly solid material lets light pass right through. Why isn’t it opaque like wood or metal?
The answer, surprisingly, dips into the fascinating realm of quantum physics – the strange, tiny world that governs how light and matter interact at the most fundamental level.
At its heart, it’s a story about light particles called photons and the electrons within the glass structure. And it’s a story where the energy levels of these tiny players just don’t quite match up.
Table of Contents
The Dance Between Light and Stuff: Why Most Materials Are Opaque
Before we dive into why glass is transparent, let’s consider why most materials aren’t. Think about a brick wall or a wooden door. When light – a stream of photons – hits these surfaces, it doesn’t just glide through.
Photons are packets of energy. And electrons, the negatively charged particles orbiting the nucleus of atoms, exist at specific, quantized energy levels. Think of these levels like steps on a ladder; an electron can only sit on a step, not in between them. To move to a higher step, an electron needs to absorb a precise amount of energy – exactly the difference in energy between its current step and the next available one.
In many materials, the electrons have available energy levels that perfectly match the energy carried by visible light photons. When a photon of the right energy comes along, an electron can absorb that energy, jump to a higher energy level, and the photon ceases to exist (its energy is transferred). This absorption happens repeatedly throughout the material, effectively “grabbing” or blocking the light from passing through. The material appears opaque or, if only certain wavelengths are absorbed, colored.
Glass: A Quantum Oddity with Wide Energy Gaps
Glass, typically made from silicon dioxide (SiO2) mixed with other compounds, is different. Unlike crystalline materials where atoms are arranged in a highly ordered, repeating pattern, glass is an amorphous solid. Its atoms are linked in a more disordered, random network, yet still maintaining the strong chemical bonds.
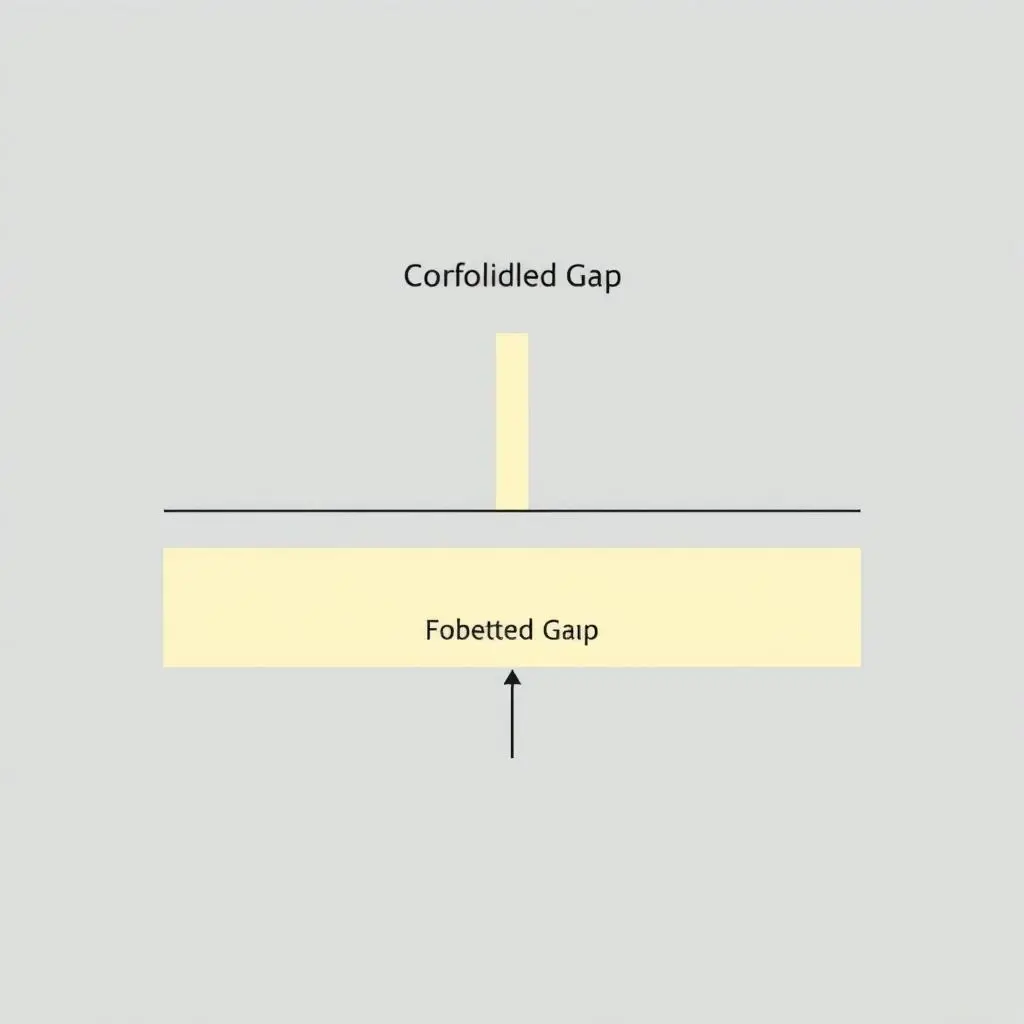
This specific atomic arrangement and the nature of the chemical bonds in SiO2 create a unique situation for its electrons and their energy levels. Instead of discrete steps, it’s more accurate to think of allowed energy bands separated by “forbidden” energy gaps where electrons simply cannot exist. The electrons normally reside in the lower energy band (the valence band).
To jump to the next allowed energy band (the conduction band), an electron needs to cross this forbidden gap. And here’s the key: in glass, this forbidden energy gap is quite large.
Why Visible Light Doesn’t Get Absorbed
Visible light – the spectrum our eyes can perceive, from red to violet – consists of photons with a range of energies. However, the energy of visible light photons is significantly *less* than the energy required for an electron in glass to jump across that large forbidden gap from the valence band to the conduction band.
It’s like trying to use a small step stool to climb over a towering wall. The photons just don’t have enough energy to be absorbed and excite the electrons to a higher state. Since the electrons can’t absorb these visible light photons, the photons continue their journey, passing straight through the material largely unimpeded.
This mismatch in energy levels is the core quantum reason behind glass transparency to visible light. The electrons in glass are simply non-reactive to the energy levels presented by visible light photons.
It’s Not Just Magic: Limits to Transparency
While incredibly transparent to visible light, glass isn’t perfectly invisible. You might notice reflections or slight distortions. Also, glass isn’t transparent to all forms of light. It readily absorbs ultraviolet (UV) light and infrared (IR) light because photons in those parts of the spectrum *do* have energies that match other possible electron transitions or molecular vibrations within the glass structure. This is why you can get a sunburn through a car window (UV-A passes, but UV-B is mostly blocked) or feel heat from the sun through a window (IR is partially absorbed).
See the Science in Action
Sometimes, seeing a quick visual explanation helps cement these concepts. Check out this short video that captures the essence of the quantum trick behind glass transparency:
Amazing how something we interact with every day is a testament to the subtle, yet powerful, rules of quantum mechanics, isn’t it?
Physics You Can Touch
Next time you look through a window, consider the invisible handshake – or rather, the missed connection – between photons and electrons happening millions of times per second. It’s a perfect, quantum mismatch that allows us to see the world outside, clear as day.
Glass transparency is a beautiful example of how fundamental physics principles directly impact the properties of materials and the technology we use (like fiber optics, which rely on glass’s transparency!).
Your Burning Questions About See-Through Stuff
Q: Does this mean all transparent materials work the same way?
A: While the principle of light passing through due to lack of absorption is similar, the specific reasons vary. Plastics, for instance, are transparent because their complex molecular structures often lack electron energy transitions corresponding to visible light energies. Water is transparent because its molecules only absorb photons outside the visible spectrum (primarily IR and UV, which is why deep water appears blue – red light is absorbed first).
Q: Why are some types of glass not perfectly clear, like frosted glass?
A: Frosted glass or other non-clear types are usually still transparent at the quantum level (photons pass through the material itself). Their lack of clarity comes from surface treatments or internal structures (like etching, sandblasting, or internal particles/bubbles) that *scatter* the light as it passes through, preventing a clear image from forming.
Q: Can we make other materials transparent?
A: Scientists are researching ways to make traditionally opaque materials transparent. This often involves manipulating their structure at the nanoscale or creating composite materials where light scattering and absorption are minimized for specific wavelengths. Think transparent ceramics or even attempts at transparent aluminum oxide (sapphire is an example, but it’s difficult to make large pieces).
Q: Is glass completely colorless because of this?
A: Pure silicon dioxide glass is inherently colorless because it doesn’t absorb visible light. However, adding certain impurities (like metal oxides) can introduce electron energy levels that *do* match visible light photons, causing specific colors to be absorbed and the glass to appear colored (e.g., iron impurities give glass a greenish tint).
A Clear View of Physics
The transparency of glass isn’t just a simple property; it’s a macroscopic manifestation of quantum rules playing out on an atomic stage. It’s a reminder that the seemingly solid, stable world we inhabit is built upon the strange and wonderful interactions of the very small. So next time you glance through a window, give a nod to the quantum physics making that view possible.